Human IL-4 人白介41600/48T; 2600/9
产品名称:Human IL-4 人白介4
产品型号:1600/48T; 2600/9
产品特点:Human IL-4 人白介4定量检测金畔生物ELISA试剂盒的优势:1,包被的酶标板单板可拆(能拆分成12个8孔的酶标条)2,提供免费代测代检服务,代出实验数据3,发文章高奖励4,磁铁可吸附式包装盒,包装精美耐用
1600/48T; 2600/9Human IL-4 人白介4的详细资料:

For research use only. Not for use in diagnostic procedures.
Human IL-4 人白介4
MANUFACTURED AND DISTRIBUTED BY:
Country︱Company: China︱Beijing Jinpan Science & Technology Co., Ltd
Address: NO.8, Liandong U Valley, Tongzhou District, Beijing, P.R.China.
TABLE OF CONTENTS
SECTION PAGE
BACKGROUND……………………………………………..………………………………..3
PRINCIPLE OF THE ASSAY…………………………………………………….……...3
TECHNICAL HINTS AND LIMITATIONS……………………………..….……..4
PRECAUTIONS………………………………………………………………………………..4
KIT COMPONENTS& STORAGE CONDITIONS………………….....………..5
OTHER SUPPLIES REQUIRED BUT NOT SUPPLIED……………..………..6
SPECIMEN COLLECTION & STORAGE………………….……………………….6
REAGENTS PREPARATION…………………………..………………………………..6
ASSAY PROCEDURE ……………………….………………………..…………………...8
CALCULATION OF RESULTS……………….……………………….………………..8
PERFORMANCE CHARACTERISTICS…………………………………………….10
REFERENCES………………………………………………………………………………….11
Human IL-4 人白介4
BACKGROUND
The interleukin 4 (IL4, IL-4) is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. The cell that initially produces IL-4, thus inducing Th0 differentiation, has not been identified, but recent studies suggest that basophils may be the effector cell.It is closely related and has functions similar to Interleukin 13.IL-4 has been shown to drive mitogenesis, dedifferentiation, and metastasis in rhabdomyosarcoma.IL-4, along with other Th2 cytokines, is involved in the airway inflammation observed in the lungs of patients with allergic asthma.
PRINCIPLE OF THE ASSAY
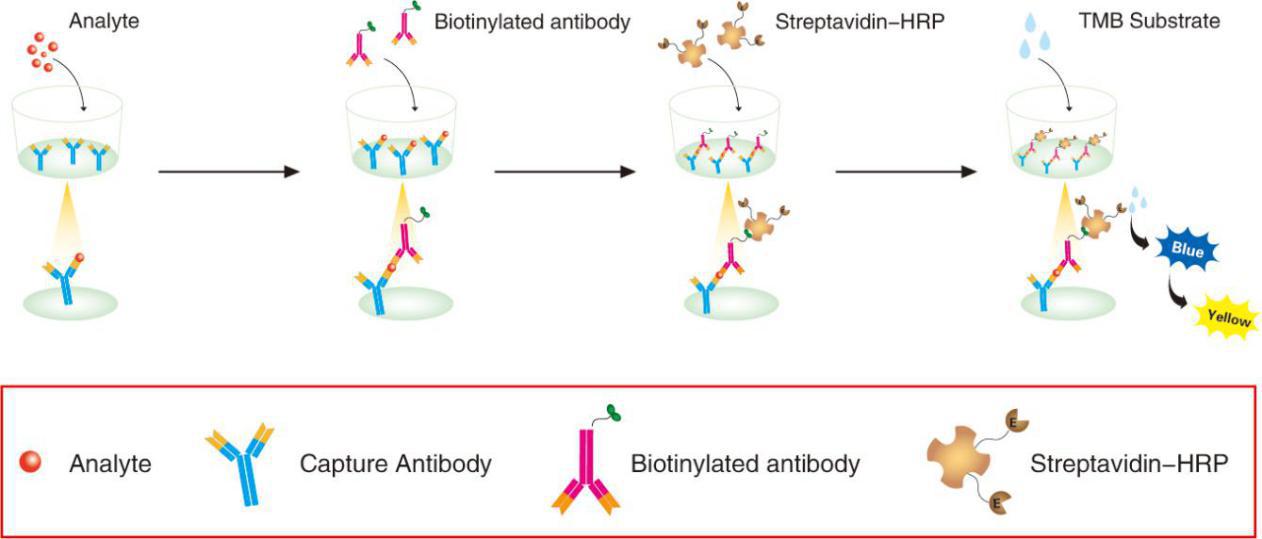
This assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for IL-4 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any IL-4 present is captured by the coated antibody after incubation. Following extensive washing, a biotin-conjugate antibody specific for IL-4 is added to detect the captured IL-4 protein in sample. For signal development, horseradish peroxidase (HRP)-conjugated Streptavidin is added, followed by Tetramethyl-benzidine (TMB) reagent. Following a wash to remove any unbound combination, and enzyme conjugate is added to the wells. Solution containing sulfuric acid is used to stop color development and the color intensity which is proportional to the quantity of bound protein is measurable at 450nm.
TECHNICAL HINTS AND LIMITATIONS
- This Jinpan ELISA should not be used beyond the expiration data on the kit label.
- To avoid cross-contamination, use a fresh reagent reservoir and pipette tips for each step.
- To ensure accurate results, some details, such as technique, plasticware and water sources should be emphasized.
- A thorough and consistent wash technique is essential for proper assay performance.
- A standard curve should be generated for each set of samples assayed.
- It is recommended that all standards and samples be assayed in duplicate.
- Avoid microbial contamination of reagents and buffers. Buffers containing protein should be made under aseptic conditions and be prepared fresh daily.
- In order to ensure the accuracy of the results, the standard curve should be made every time.
PRECAUTIONS
The Stop Solution suggested for use with this kit is an acid solution. Wear protective gloves, clothing, eye, and face protection. Wash hands thoroughly after handling.
KIT COMPONENTS& STORAGE CONDITIONS
|
PART |
SIZE |
STORAGE OF OPENED/ RECONSTITUTED MATERIAL |
|
Microwell Plate – antibody coated 96-well Microplate (8 wells ×12 strips) |
1 plate |
Return unused wells to the foil pouch containing the desiccant pack. Reseal along entire edge of the zip-seal. May be stored for up to 1 month at 2 – 8℃** |
|
Standard – lyophilized,1000 pg/ml upon reconstitution |
2 vials |
Aliquot and Store at -20°C** for six months |
|
Concentrated Biotin-Conjugated antibody(100X) – 120 ul/vial |
1 vial |
Store at 2-8°C **for six months |
|
Concentrated Streptavidin-HRP solution(100X) – 120 ul/vial |
1 vial |
Store at 2-8°C** for six months |
|
Standard /sample Diluent – 16 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Biotin-Conjugate antibody Diluent – 16 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Streptavidin-HRP Diluent – 16 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Wash Buffer Concentrate (20x) – 30 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Substrate Solution – 12 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Stop Solution – 12 ml/vial |
1 bottle |
Store at 2-8°C** for six months |
|
Plate Cover Seals |
4 pieces |
|
**Provided this is within the expiration date of the kit.
OTHER SUPPLIES REQUIRED BUT NOT SUPPLIED
- Microplate reader capable of measuring absorbance at 450 nm.
- Pipettes and pipette tips.
- Deionized or distilled water.
- Squirt bottle, manifold dispenser, or automated microplate washer.
- 500 mL graduated cylinder.
- Human IL-4 controls (optional; available from Jinpan).
SPECIMEN COLLECTION & STORAGE
Cell Culture Supernates – Centrifuge cell culture media at 1000×g to remove debris. Assay immediay or aliquot and store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Serum – Use a serum separator tube (SST) and allow samples to clot for 2 hours at room temperature or overnight at 2-8℃. Centrifuge at approximay for 15 minutes at 1000×g. Assay immediay or aliquot and store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Plasma – Collect plasma using EDTA, heparin, or citrate as an anticoagulant. Centrifuge for 15 minutes at 1000×g within 30 minutes of collection. Assay immediay or aliquot and store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Note: The normal human serum or plasma samples are suggested to make a 1:2 dilution.
REAGENTS PREPARATION
- Temperature returning – Bring all kit components and specimen to room temperature (20-25℃) before use.
- Wash Buffer – Dilute 30mL of Wash Buffer Concentrate with 570mL of deionized or distilled water to prepare 600mL of Wash Buffer. If crystals have formed in the concentrate Wash Buffer, warm to room temperature and mix gently until the crystals have compley dissolved.
- StandardSpecimen – Reconstitute the Standard with 1.0mL of deionized or distilled water. This reconstitution produces a stock solution of 1000pg/mL. Allow the standard to sit for a minimum of 15 minutes with gentle agitation prior to making dilutions. Pipette 800mL of Standard/Specimen Diluent into the 200 pg/mL tube, and add 200mL stock solution of 1000 pg/mL into it to get the high standard of 200 pg/mL. Pipette 500mL of Standard/Specimen Diluent into the remaining tubes. Use the high standard to produce a 2-fold dilution series (below).. Mix each tube thoroughly and change pipette tips between each transfer. The 200pg/mL standard serves as the high standard. The Standard/specimen Diluent serves as the zero standard (0pg/mL).
*If you do not run out of re-melting standard, store it at -20℃. Diluted standard shall not be reused.
- Working solution of Biotin-Conjugate anti-human IL-4 antibody: Make a 1:100 dilution of the concentrated Biotin-Conjugate solution with the Biotin-Conjugate antibody Diluent in a clean plastic tube.
*The working solution should be used within one day after dilution.
- Working solution of Streptavidin-HRP: Make a 1:100 dilution of the concentrated Streptavidin-HRP solution with the Streptavidin-HRP Diluent in a clean plastic tube.
*The working solution should be used within one day after dilution.
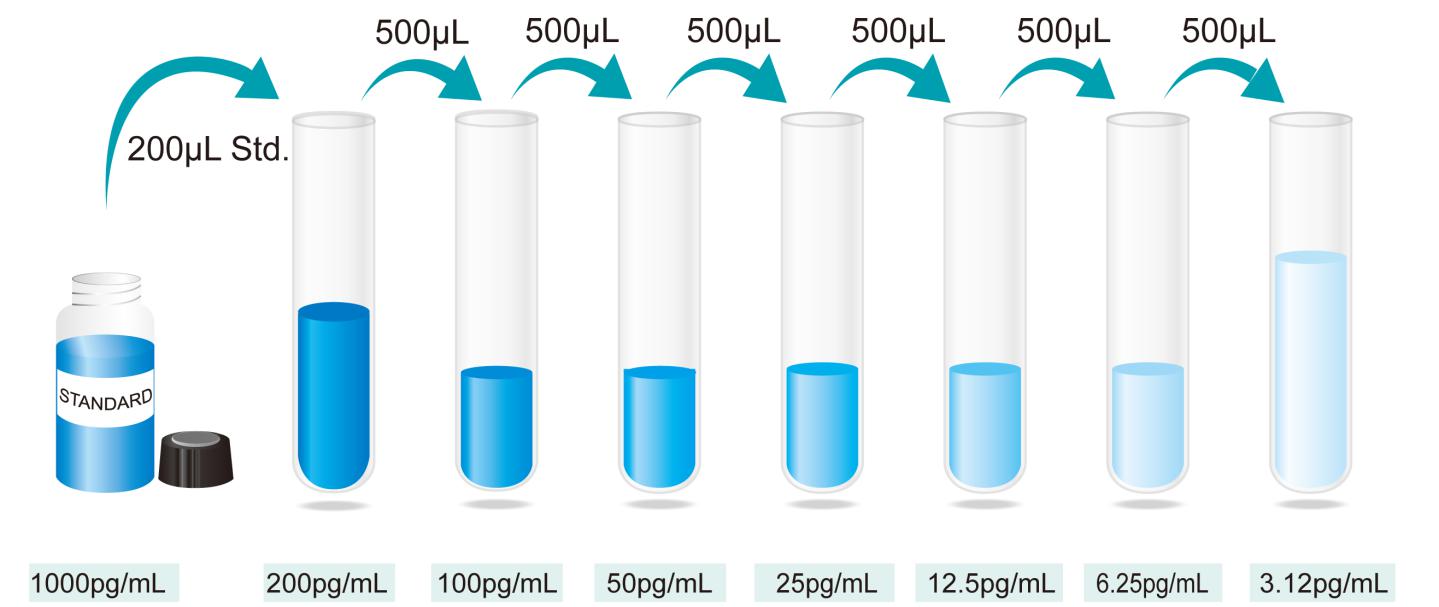
Preparation of IL-4 standard dilutions
ASSAY PROCEDURE
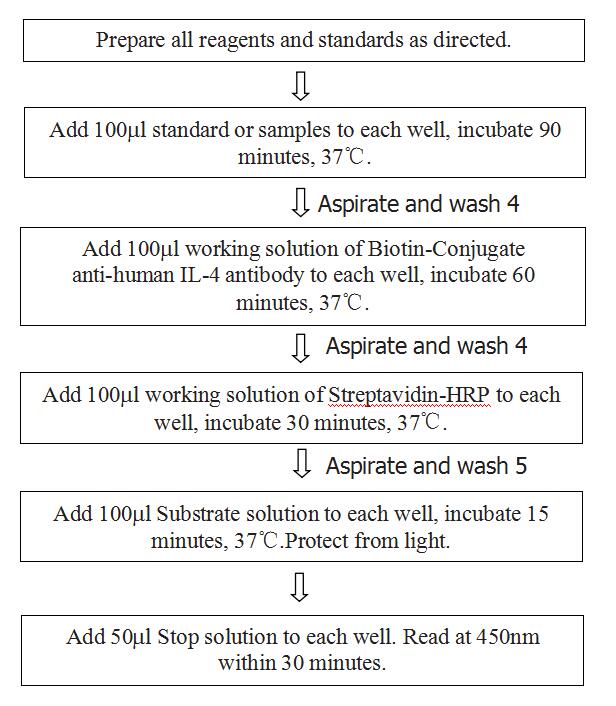 CALCULATION OF RESULTS
CALCULATION OF RESULTS
- The standard curve is used to determine the amount of specimens.
- First, average the duplicate readings for each standard, control, and sample. All O.D. values are subtracted by the mean value of blank control before result interpretation.
- Construct a standard curve by reducing the data using computer software capable of generating a four parameter logistic (4-PL) curve-fit. As an alternative, construct a standard curve by plotting the mean absorbance for each standard on the y-axis against the concentration on the x-axis and draw a best fit curve through the points on the graph.
- The data may be linearized by plotting the log of the IL-4 concentrations versus the log of the O.D. and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data. If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.
- This standard curve is provided for demonstration only. A standard curve should be generated for each set of samples assayed.
Typical data using the IL-4 ELISA
|
Standard(pg/ml) |
OD. |
OD. |
Average |
Corrected |
|
0 |
0.044 |
0.04 |
0.042 |
—— |
|
3.12 |
0.097 |
0.092 |
0.0945 |
0.0525 |
|
6.25 |
0.13 |
0.106 |
0.118 |
0.076 |
|
12.5 |
0.229 |
0.233 |
0.231 |
0.189 |
|
25 |
0.42 |
0.412 |
0.416 |
0.374 |
|
50 |
0.741 |
0.734 |
0.7375 |
0.6955 |
|
100 |
1.315 |
1.306 |
1.3105 |
1.2685 |
|
200 |
2.205 |
2.212 |
2.2085 |
2.1665 |
Representative standard curve for IL-4 ELISA.
Performance Characteristics
SENSITIVITY: The minimum detectable dose was 1.5pg/mL.
SPECIFICITY: This assay recognizes both natural and recombinant human IL-4. The factors listed below were prepared at 100ng/ml in Standard /sample Diluent and assayed for cross-reactivity and no significant cross-reactivity or interference was observed.
Factors assayed for cross-reactivity
|
Recombinant human |
Recombinant mouse |
Recombinant porcine |
|
G-CSF |
IL-1 |
bovine FGF acidic |
|
GM-CSF |
IL-4 |
bovine FGF basic |
|
IL-1α_ |
IL-3 |
human PDGF |
|
IL-1β_ |
IL-4 |
porcine PDGF |
|
IL-2 sRα_ |
IL-5 |
|
|
IL-3 |
|
|
|
IL-4 |
|
|
|
IL-6 |
|
|
|
IL-7 |
|
|
|
IL-8 |
|
|
|
LIF |
|
|
|
TGF-β1 |
|
|
|
TGF-β2 |
|
|
REPEATABILITY: The coefficient of variation of both intra-assay and inter-assay were less than 10%.
RECOVERY:The recovery of IL-4 spiked to three different levels in four samples throughout the range of the assay in various matrices was evaluated.
Recovery of IL-4 in two matrices
|
Sample Type |
Average % of Expected Range (%) |
Range (%) |
|
Citrate plasma |
88 |
82-94 |
|
Cell culture supernatants |
97 |
92-102 |
LINEARITY:To assess the linearity of the assay, three samples were spiked with high concentrations of IL-4 in various matrices and diluted with the appropriate Sample Diluent to produce samples with values within the dynamic range of the assay.
The linearity of the assay
|
Dilution ratio |
Recovery (%) |
Citrate plasma |
Cell culture supernatants |
|
1:2 |
Average% of Expected |
85 |
96 |
|
Range (%) |
81–89 |
92-100 |
|
|
1:4 |
Average% of Expected |
92 |
102 |
|
Range (%) |
87–97 |
96-108 |
REFERENCES
- Sokol, C.L., Barton, G.M., Farr, A.G. & Medzhitov, R. (2008). “A mechanism for the initiation of allergen-induced T helper type 2 responses”. Nat Immunol 9 (3): 310–318.
- Liang, H-E, et al. (2012) Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature Immunology, 13: 58–66.
- Howard M, Paul WE (1982). “Interleukins for B lymphocytes”. Lymphokine Res. 1 (1): 1–4.
- Yokota T et al. (1986). “Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that express
